DISPLAY 101
#26 Fluorescence and Phosphorescence
The best feature of organic light-emitting diodes (OLEDs) is that each diode emits light on its own. Let’s recap how OLED works.
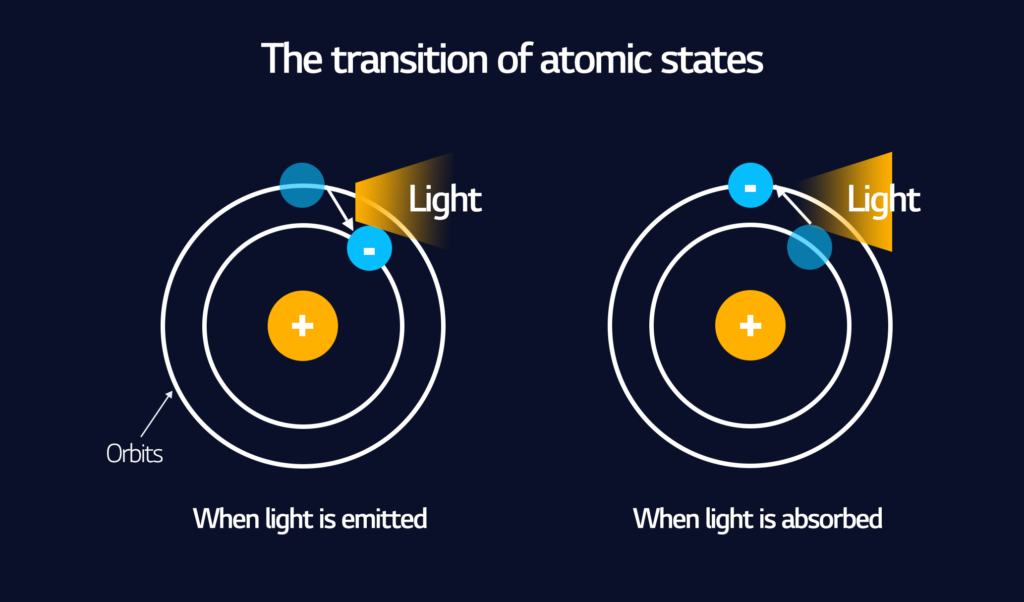
In OLED, light is emitted on an atomic level from the electrons of individual atoms. Electrons have general areas where they can be found in an atom, called “orbitals.” Each orbital can fit a specific, even number of electrons in it. The smallest atom can have two electrons in one orbital, but larger atoms have more electrons in an outer orbital.
Energy hitting an atom can cause electrons to move into an outer orbital. Then, they spontaneously give off energy and move back down to an inner orbital. When that energy is visible to the naked eye, we call it light.
What makes OLED substances unique is that they give off visible light in this fashion when they are excited by energy. Also, they easily accept energy in the form of electricity, which is a convenient feature for electronic products such as displays.
But there are actually two ways electrons give off visible light: fluorescence and phosphorescence. What are the similarities and differences between them? Let’s find out below.
Two similar yet distinct emission methods
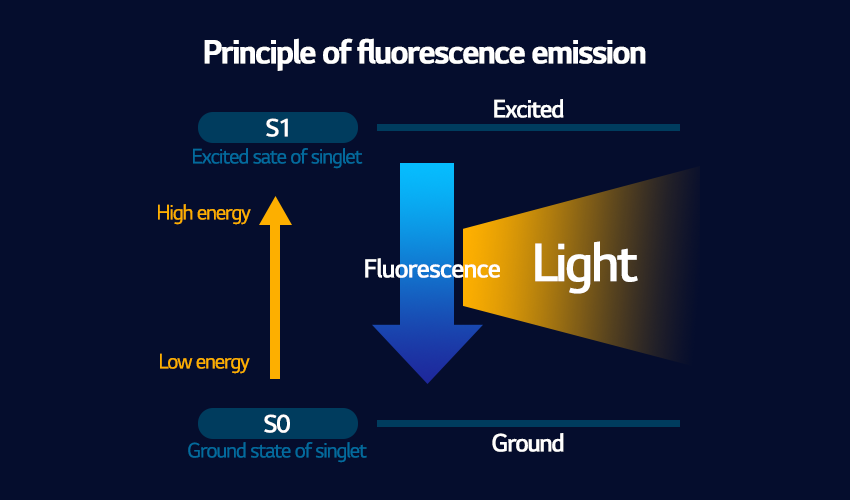
Fluorescence is a type of bright light emission that starts and stops immediately depending on the energy source. As soon as energy is absorbed by the atoms they emit light, meaning that the excited atom and its electrons have a very short lifetime before they transition back to a low energy state. Fluorescence is a very simple transition from a high level (an excited state) to a low level (a ground state), as shown in the diagram above. Also, the emitted light always has a longer wavelength than the absorbed energy, and hence will always be of lower energy than what was absorbed. In practical terms, there is an immediate flash or afterglow that can be seen when the substance is excited.
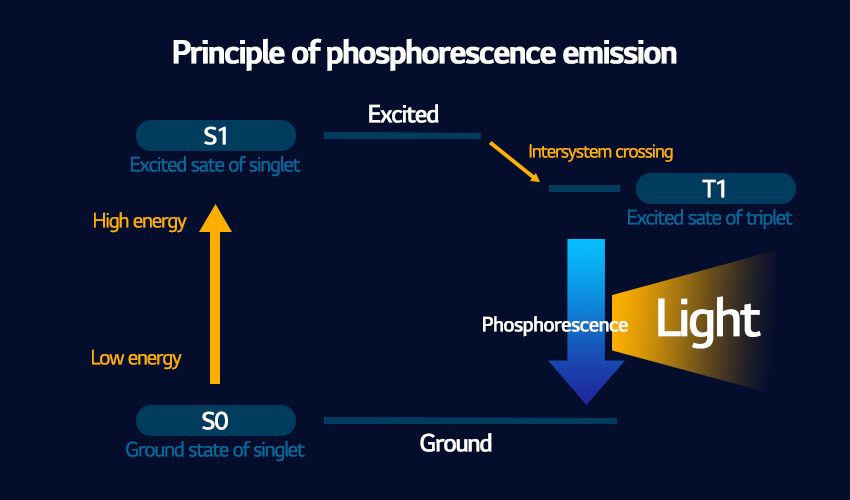
Phosphorescence pertains to light that grows slowly as it receives energy and also continues to glow for some time even after the energy is no longer supplied. The excited electrons in these atoms go through a comparatively longer life cycle before they transition back to a low energy state. The transition between the higher energy state and the lower energy state is much more complicated in a phosphorescent substance, which is why it takes so long to happen compared to fluorescence. This is shown in the diagram above, where an additional step from a S1 to a T1 state called ‘intersystem crossing’ takes place. Just like with fluorescence, the light that is emitted is lower energy than what was absorbed, but it is actually even lower energy, and even a longer wavelength, than what fluorescence gives off. Because of this lower emission of light over a longer period of time, phosphorescent materials often appear to glow in the dark without having an energy source.
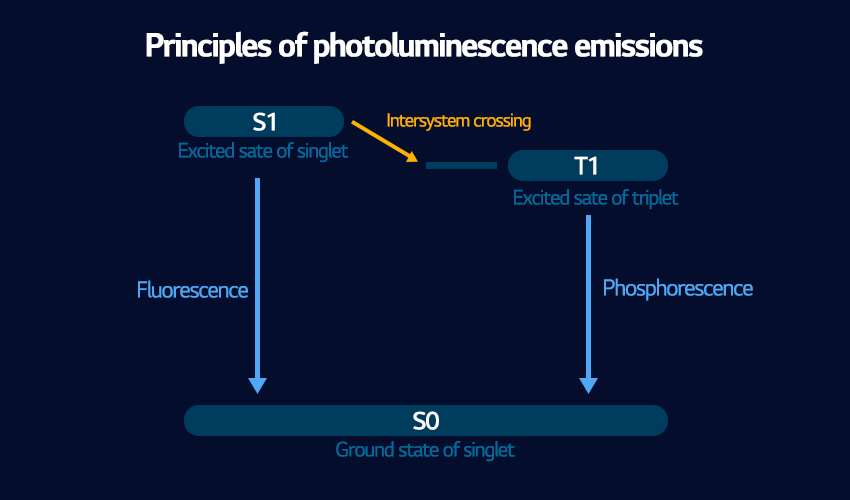
Together, these two methods are called ‘photoluminescence’, and both of them are used for OLED panels. But why two methods? And what’s the difference between them? Something bigger and more complicated must be happening with phosphorescence to make it take so much more time and end up with less energy than fluorescence. It turns out that the answer is complicated.
In phosphorescence, the electrons do much more than they do in fluorescence. In fluorescence the electrons only move from one orbital to another, an action that is relatively quick and doesn’t cost that much energy. But in fluorescence, the specific details of what the electrons do, the intersystem crossing, involve getting into the details of quantum mechanics and electron spin, which is a little beyond the scope of this article. In short, they take a longer journey and more of their basic properties are temporarily modified. But in the end, they go back to their original state, and the overall result is less bright light over a longer period of time.









